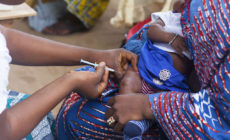
The WHO said it was ethical in light of the scale of the outbreak and high number of deaths – over 1,000 people have died in West Africa.
The statement was made after its medical experts met in Switzerland on Monday to discuss the issue.
The move came as Liberia said it was getting an experimental drug, Zmapp, after requests to the US government.
The WHO said where experimental treatments are used there must be informed consent and the results of the treatment collected and shared.
In a statement, it said: “In the particular circumstances of this outbreak, and provided certain conditions are met, the panel reached consensus that it is ethical to offer unproven interventions with as yet unknown efficacy and adverse effects, as potential treatment or prevention.”
But the organisation conceded there were still many questions to be answered including how data could be gathered effectively while the focus remained on providing good medical care.
It was also unclear where the funding for the treatment would come from.
Last week the WHO declared the Ebola outbreak was a global health emergency.
Meanwhile, the Liberian Government said experimental drugs will be brought into the country later this week – although manufacturer Mapp Biopharmaceutical warned supplies are limited.
Zmapp has been used on two US aid workers who have shown signs of improvement, although it is not certain what role the medication played in this.
A Roman Catholic priest, infected with Ebola in Liberia, who died after returning home to Spain is also thought to have been given the drug.
However, the drug has only been tested on monkeys and has not yet been evaluated for safety in humans.










 (Selorm) |
(Selorm) |  (Nana Kwesi)
(Nana Kwesi)