Coronavirus: Oxford University vaccine trial paused after participant falls ill
- Posted on
- Comment
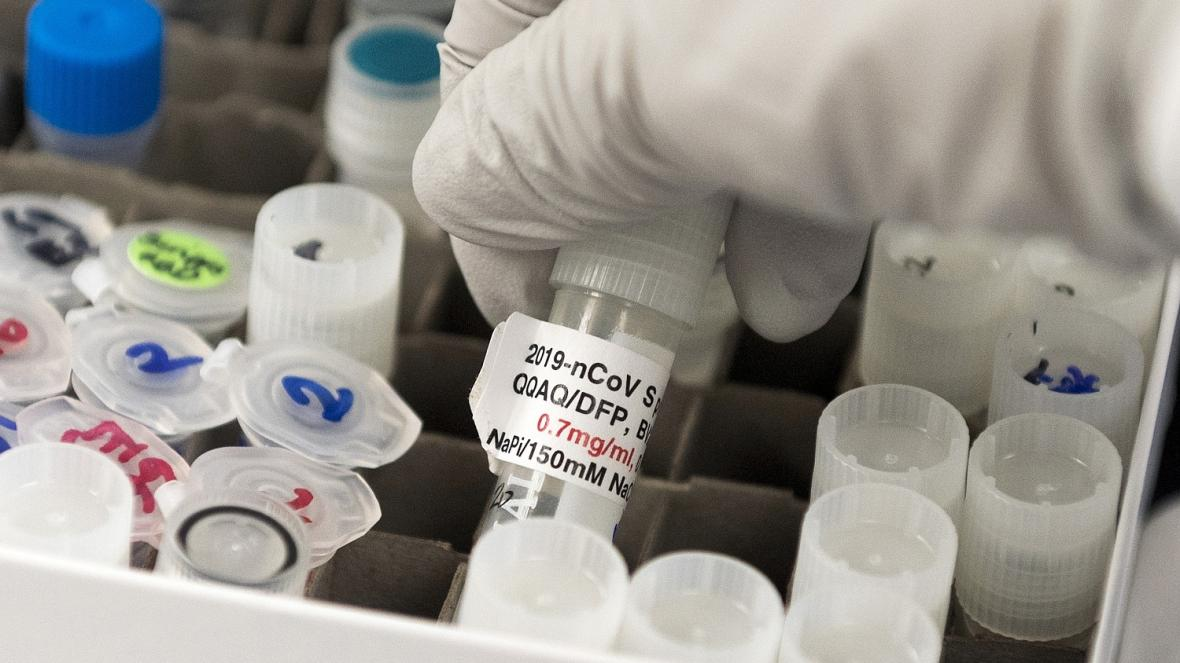
Final clinical trials for a coronavirus vaccine, developed by AstraZeneca and Oxford University, have been put on hold after a participant had a suspected adverse reaction in the UK.
AstraZeneca described it as a “routine” pause in the case of “an unexplained illness”.
The outcome of vaccine trials is being closely watched around the world.
The AstraZeneca-Oxford University vaccine is seen as a strong contender among dozens being developed globally.
Hopes have been high that the vaccine might be one of the first to come on the market, following successful phase 1 and 2 testing.
Its move to Phase 3 testing in recent weeks has involved some 30,000 participants in the US as well as in the UK, Brazil and South Africa. Phase 3 trials in vaccines often involve thousands of participants and can last several years.
The New York Times is reporting a volunteer in the UK trial has been diagnosed with transverse myelitis, an inflammatory syndrome that affects the spinal cord and can be caused by viral infections.
However, the cause of the illness has not been confirmed and an independent investigation will now work out if there was any link to the vaccine.
-BBC







 (Selorm) |
(Selorm) |  (Nana Kwesi)
(Nana Kwesi)