Covid-19: China’s Sinovac vaccine trial halted in Brazil
- Posted on
- Comment
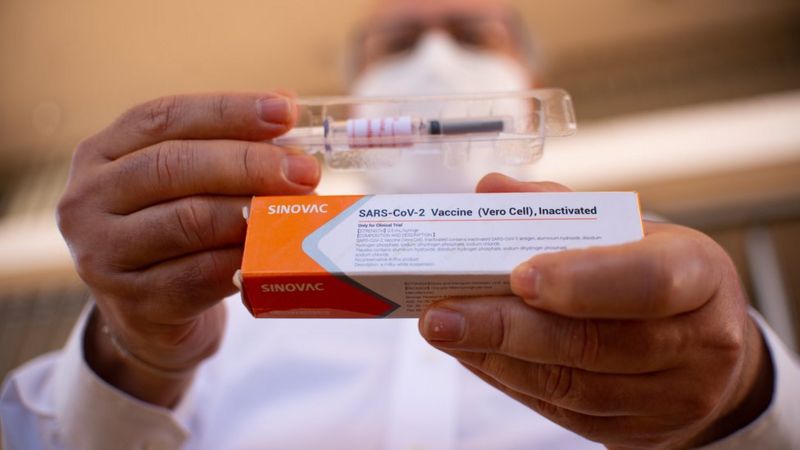
The Brazilian clinical trial for a Chinese Covid-19 vaccine has been suspended after health authorities reported a “severe adverse” incident.
Brazilian health regulator Anvisa said the incident took place on 29 October, but did not give further details.
The CoronaVac vaccine, developed by the Chinese firm Sinovac Biotech, is one of several in final-stage testing globally.
Sinovac says it is “confident in the safety of the vaccine”.
The firm has already been using it to immunise thousands of people at home in an emergency use programme.
Brazil has been one of the countries worst affected by coronavirus, recording more than 5.6m confirmed cases – the third highest tally in the world after the US and India – and nearly 163,000 deaths so far, according to data collated by Johns Hopkins University.
Why was the trial halted?
On Monday Anvisa said it had “ruled to interrupt the clinical trial of the CoronaVac vaccine after a serious adverse incident”.
It did not reveal what happened, nor where it took place. Late-stage trials for the Sinovac vaccine are also being conducted in Indonesia and Turkey, but neither of these countries have announced a suspension.
Indonesia’s state-owned Bio Farma said on Tuesday that its own Sinovac vaccine trials were “going smoothly”, according to Reuters news agency.
Dimas Covas, the head of Butantan, the medical research institute conducting the Brazilian trial, told local media that the trial’s suspension was related to a death. However, he insisted that the death was not related to the vaccine, Reuters said.
Sinovac said on Tuesday that it was communicating with Brazil about the reported incident.
“We learned the head of Butantan Institute believed that this serious adverse event (SAE) is not related to the vaccine,” it said in a statement. “The clinical study in Brazil is strictly carried out in accordance with GCP (Good Clinical Practice) requirements and we are confident in the safety of the vaccine.”
Butantan has said it will hold a news conference on Tuesday at 11:00 local time (14:00 GMT).
A pause in a clinical trial is not unusual. In September, the UK paused trials for another Covid-19 vaccine after a participant had a suspected adverse reaction.
The trails for the vaccine being developed by AstraZeneca and Oxford University were resumed a few days later after regulators said it was safe to continue.
Brazil’s President Jair Bolsonaro has been open about his preference for the vaccine being developed by AstraZeneca, saying his government would not buy a Chinese-made Covid-19 vaccine.
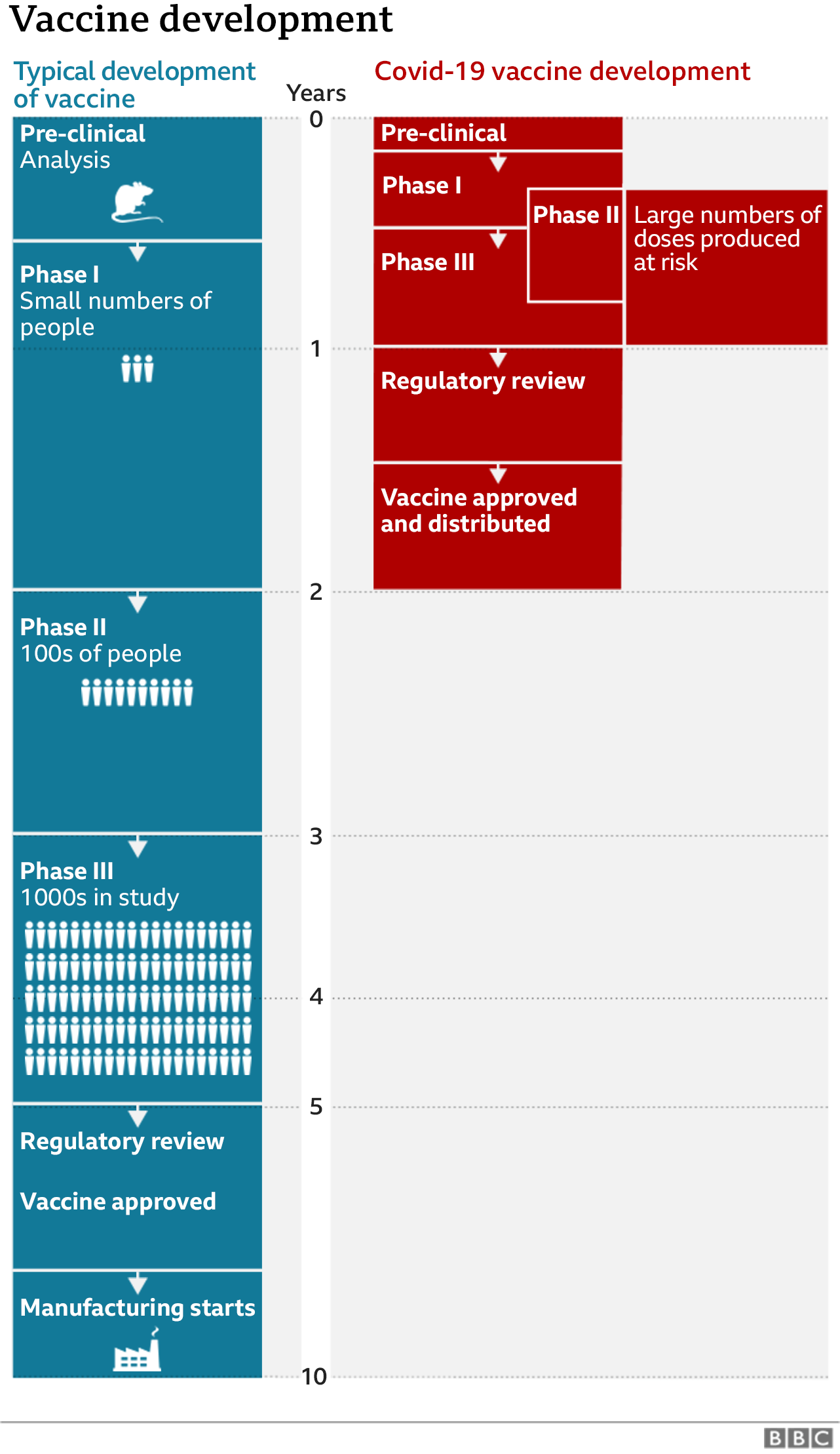
Where are we in the search for a vaccine?
CoronaVac is one of around a dozen vaccines in the final stage of testing – known as a phase 3 trial – around the world.
This is a crucial point in vaccine development, where some experimental vaccines will fail.
The news of its suspension in Brazil came shortly after a rival vaccine developer, the US pharmaceutical company Pfizer, said its own vaccine canidate had shown 90% effectiveness.
Last month the Oxford vaccine trial reviewed the death of a volunteer in Brazil, saying an assessment had revealed no safety concerns.
How has China used experimental vaccines?
Separate to the phase 3 trials being held overseas, China is also administering experimental Covid-19 vaccines at home.
CoronaVac is among three experimental coronavirus vaccines China has been using to inoculate hundreds of thousands of people under an emergency use programme.
Last month the BBC filmed hundreds of people in the city of Yiwu queuing to get the vaccine after authorities approved the distribution to anyone who wanted the injection.
A businessman who is due to have the second of the required two jabs imminently told the BBC that he would go ahead with it, adding that it is “worth it considering the high infection rate abroad”.
Sinovac has previously said almost all of its employees and their families have received the vaccine.
And a Chinese health official earlier said that serious side effects have not been observed in clinical trials.
-BBC










 (Selorm) |
(Selorm) |  (Nana Kwesi)
(Nana Kwesi)